Frequently Asked Questions
| Q: |
What is Birmingham Quality and what does it do? |
| A: |
Birmingham Quality is the largest of the UK NEQAS centres providing External Quality Assessment (EQA) services in Clinical Chemistry. We also provide some non-UK NEQAS EQA programmes that are delivered to the same high standards as UK NEQAS services. Birmingham Quality is an NHS-based department located at the University Hospitals Birmingham NHS Foundation Trust For more information about Birmingham Quality, please see About Us |
| Q: |
What is UK NEQAS and what does it do? |
| A: |
UK NEQAS is the United Kingdom External Quality Assessment Service which is a charitable consortium of External Quality Assessment Laboratories / Centres. There are a number of UK NEQAS centres around the UK providing External Quality Assessment services in many different disciplines. Birmingham Quality is the largest of the centres providing primarily chemistry Schemes. For more information about the UK NEQAS organisation, please see https://ukneqas.org.uk/about-us/ For more information about the different UK NEQAS centres and the EQA programmes they provide, please see https://ukneqas.org.uk/our-centres/ |
| Q: |
When do I use my UK NEQAS ID / Laboratory number / Account number? |
| A: |
This is probably the most important of our FAQs. All your Reports and Request Cards / Results Documents use your UK NEQAS ID as the index to all your data. This is fundamental to the way we operate. You must quote your UK NEQAS ID in all correspondence. This is essentially your Account Number. Without this we cannot handle your enquiry. Many physical laboratories have more than one UK NEQAS ID. This could be another analyser or another site. In the same way that Patients are often identified by two-out-of-three of Name, Date-of-Birth or Hospital or NHS number, we need to be sure who we are dealing with. We may ask you supplementary questions if you are phoning. If you email us, please don't assume that we know who you are just by virtue of your footer. You must quote the UK NEQAS ID to which your enquiry refers. If it is a general enquiry you can just quote you 'main' UK NEQAS ID. If you are changing things for a particular analyser or site you must be crystal clear as to which UK NEQAS ID these changes refer. Unfortunately, saying 'all of them' isn't sufficient for us to act on your request. Sorry. All participants use their UK NEQAS laboratory identifier as username, and a password which is notified to them when they join. Most Participants have been with us for years and will have some local internal SOP / Document that contains this information. Because a Laboratory Account is accessed by multiple individuals, it is better to ask your colleagues for your local approach if you lose / forget passwords because if we change your log-in password for you personally, it will be up to you to share this with rest of your Department. Your password can be changed to something more memorable and can be changed by you, the participant. Please contact Birmingham Quality by email if you require further information or re-notification of your password. |
| Q: |
How do I pay for my EQA services? |
| A: |
It comes as a shock to many Participants that they have to pay for their EQA services. We are an NHS Department in an NHS Hospital and our only, repeat only, source of funding is from subscription income for the services we provide. At the start of the Calendar Year we open our on-line registration. By the beginning of the Financial Year on 1st April, Participants will have agreed what they want and we will be able to provide a formal quotation to allow Participants to raise a Purchase Order number. If you have made no changes, you could provide a Purchase Order number based on the on-line amount. If you have made changes, we will incorporate them into a formal quote. There really is no reason why you shouldn't be able to give us a Purchase Order number in April. Our overseas Participants may be used to a more forgiving system than we are obliged to use as an NHS Department and though VAT may have to be paid you may only need a 'reference' number rather than a Purchase Order number which simplifies things. Our primary contact is through you, the Laboratory Participant. We will follow up problems with you, as you are in the best place to cope with the vagaries of your own Finance Department. Just because you have forwarded something to your own Finance Department, this does not mean it will have been actioned and the money has come to us or to our Finance Department. Participants are beginning to expect an 'Amazon' experience. There are two problems with this laudable aspiration:
|
| Q: |
How do I get access to the Wolfson UK NEQAS results and reports web service? |
| A: |
The service is available for all Birmingham Quality UK NEQAS services, and UK NEQAS services at Glasgow (Cardiac Markers), Edinburgh (Peptide Hormones), Guildford (Peptide Hormones & Trace Elements), Colindale (Microbiology), Bristol (Antibiotic Assays), Manchester (Reproductive Science) and Parasitology. All participants use their UK NEQAS laboratory identifier code as username, and a password which is notified to them with the web service documentation. This can then be changed by the participant. Please contact Birmingham Quality by email if you require further information or renotification of your password. |
| Q: |
I am registered for the Wolfson UK NEQAS results and reports web service but have forgotten my password |
| A: |
Please contact Birmingham Quality by email with Password reset request in the subject line, requesting renotification of your password. It is essential to include the UK NEQAS laboratory identifier code (s) concerned, your name, and your laboratory address for verification purposes. Your request will be dealt with in the next batch of renotifications (usually within one working day). |
| Q: |
I am registered for the Wolfson UK NEQAS results and reports web service but cannot seem to log in with the password provided |
| A: |
|
| Q: |
Against which ISO standard is Birmingham Quality accredited? |
| A: |
University Hospitals Birmingham NHS Foundation Trust operating Birmingham Quality is accredited to ISO 17043:2023 General Requirements for Proficiency Testing. University Hospitals Birmingham NHS Foundation Trust operating Birmingham Quality is a UKAS Accredited Proficiency Testing Provider, Number 7860. Our schedule of accreditation can be viewed in the Directory of Accredited Organisations on the UKAS website here. |
| Q: |
Why should I choose Birmingham Quality services over those of another EQA provider? |
| A: |
Birmingham Quality as part of the wider UK NEQAS organisation, has over 50 years’ experience in providing high quality EQA services for Clinical Biochemistry. We do not offer one-size-fits-all or no-frills services but are instead, committed to ensuring education and training are integral to our comprehensive and ever-expanding range of EQA programmes. Each year, we conduct a variety of different value-added studies across all of our EQA programmes examining everything from interfering substances and disease states that can adversely affect your assay results, to potential complications from pre- and post-analytical technique. We also undertake regular surveys exploring topics relevant to assay quality such as reference ranges and interpretative elements. Findings from our studies are fed back to Participants in real time via commentaries and special reports; delivering the information you need to know, without delay. Our workshops, roadshows and webinars run throughout the year, giving you access to the latest scientific and educational updates from Birmingham Quality that you can employ to continually improve your Clinical Biochemistry service. When you sign up to become a member of our services, you also have unlimited† access to advice and data from our very experienced and expert consultant scientists. All of our services are designed with you, our users, at heart. We are here to make sure that your assay knowledge is the best it it can be; enabling you to deliver the best possible service to your users. That's why we work with you to provide the best service for you. We also liaise closely with with manufacturers, regulatory and professional bodies to ensure that they also are fully aware of the advantages and limitations of different laboratory assays. Where else can you gain access to all of the thorough, timely and relevant information you need to be the best at what you do? Why not make EQA more than just a box ticking exercise and sign up today? †a fair usage policy applies! |
| Q: |
How do I register to become a participant in a Birmingham Quality EQA programme? |
| A: |
Detailed information about each of the individual EQA programmes provided by Birmingham Quality may be found: https://birminghamquality.org.uk/eqa-programmes/ Each individual programme page features a “Sign up today” button. Please click on the Sign up today button, complete your details and click on Submit. Birmingham Quality will respond to your enquiry within 2 business days. You can also sign up by getting in touch by telephone on +44 (0) 121 414 7300 or by emailing birminghamquality@uhb.nhs.uk |
| Q: |
It is nearing the end of the financial year. What do I need to do to remain registered as a participant in Birmingham Quality’s EQA programmes for the coming year? |
| A: |
As the end of each financial year approaches, we open up our online re-registration service and formally invite Participants to review and update their participation in the EQA programmes provided by Birmingham Quality. Online re-registration remains open online for a number of weeks and we contact Participants at regular intervals throughout this period to remind them that the re-registration deadline is approaching and that action is required. When the re-registration period ends, Birmingham Quality will use the information you gave to produce a quotation for the EQA services it will be providing to your organisation for the following year. Participants can then use the online re-registration service to provide us with a Purchase Order number to cover the quotation and which will enable our host Trust (University Hospitals Birmingham NHS Foundation Trust) to generate an invoice for your Finance Department. If you do not update your organisation's EQA requirements during the re-registration period, your quotation will be based on all services in which your organisation is enrolled at the point that online re-registration closes. |
| Q: |
I no longer wish to participate in a Birmingham Quality EQA programme. What do I need to do? |
| A: |
Birmingham Quality operates its services on an annual basis; payments for EQA programmes cover the period between 1st April and 31st March inclusive. You can withdraw from any or all of the EQA programmes in which your organisation participates at any point during the year by contacting Birmingham Quality 'in writing' using the online contact form or by email at birminghamquality@uhb.nhs.uk. We regret that we are unable to accept requests of this nature by telephone. Please note that refunds are not issued to organisations withdrawing from EQA programmes part way through the year. Participants are also given the opportunity to withdraw from EQA services which they no longer require during the online re-registration process undertaken at the end of each financial year. |
| Q: |
Where an analyte is available in more than one of Birmingham Quality's EQA programmes, do I need to join all of the programmes in which the analyte appears? |
| A: |
Some analytes appear in more than one of the EQA programmes operated from Birmingham Quality. For example, Lithium is available within the UK NEQAS for Clinical Chemistry and also in the Therapeutic Drug Monitoring (TDM) section of the UK NEQAS for Tox and TDM. It is not necessary for you to participate in both services in order to externally assess the quality of the measurement of this analyte in your laboratory. Similarly, if you do participate in more than one programme offering the same analyte, you can choose not to report the analyte in multiple programmes. However, please be aware that this will not reduce the cost of the service. Please be aware that detailed educational exercises examining the analyte in detail (such as recovery and interference studies) will be undertaken in the more specialised programme. |
| Q: |
I can no longer access my Reports, but I am still getting specimens, why is this? |
| A: |
The most likely reason for this is that your laboratory has not paid for the services that you are receiving. You will have received multiple reminder emails and the switching off of the reports is a last resort. Please contact us to discuss this further. |
| Q: |
I currently have three registrations with you under Laboratory Codes 12345, 12345A and 12345B. I am replacing all analysers. What should I do with regards to Laboratory Codes? |
| A: |
As a general point of policy you do not need to change Laboratory Codes when you change your analyser. The Laboratory Code is really a ‘Location/Address/Entity’ reference similar to a customer number.
You need to maintain your Laboratory Codes as they are so that you will be able to take advantage of dashboards when they are rolled out in the near future. |
| Q: |
I am responsible for monitoring EQA performance over several sites / devices. Can I get a combined report covering all of these? |
| A: |
We offer network reports for several of our services at no additional cost. Network reports enable a co-ordinator to review the EQA performance for all sites / devices / analysers for which they are responsible, in one report. We can identify the members of your network by a short mnemonic which may be a location / device name. |
| Q: |
I am a Point of Care Co-ordinator. Can I get a combined report covering all of the devices we have registered in an EQA service? |
| A: |
Birmingham Quality offers a wide range of EQA services to POCT users. Network reports are provided free-of-charge to Point-of-Care co-ordinators enabling them to review the EQA performance for all sites / devices / analysers for which they are responsible, in one report. We can identify the members of your network by a short mnemonic which may be a location / device name. |
| Q: |
How are EQA specimens sent to participants in the UK? |
| A: |
Birmingham Quality routinely dispatches EQA specimens to UK-based participants via Royal Mail. Specimens will be dispatched to participants outside of the UK via standard airmail. We also offer the facility to deliver EQA specimens by courier. Two options are available. We can either:
Please contact us to set up courier delivery. If you wish to use your own courier, please supply us with your courier's contact details and your account number. Please note that there will be a minimum period that you have to commit to. |
| Q: |
How are EQA specimens sent to participants outside of the UK? |
| A: |
Birmingham Quality routinely sends EQA specimens to participants via standard airmail. We also offer the facility to deliver EQA specimens by courier. Two options are available. We can either:
Please contact us to set up courier delivery. If you wish to use your own courier, please supply us with your courier's contact details and your account number. Please note that there will be a minimum period that you have to commit to; notionally until 31st March of the current financial year. |
| Q: |
Can you send EQA specimens by courier? |
| A: |
Yes, we offer the facility to deliver EQA specimens by courier. Two options are available. We can either:
Please contact us to set up courier delivery. If you wish to use your own courier, please supply us with your courier's contact details and your account number. Please note that there will be a minimum period that you have to commit to; notionally until 31st March of the current financial year. |
| Q: |
Why are there different numbers of specimens in different EQA programmes? |
| A: |
The default position is three specimens for our regular Schemes and two specimens for our POCT Schemes. Some of our Schemes have individual specimens for an analyte where is is not possible to cover all concentrations with a single sample. |
| Q: |
Why does the frequency of specimen dispatch differ between EQA programmes? |
| A: |
Most of our services are issued notionally 'monthly' and we tend to issue 11 distributions a year taking into account Christmas and Easter etc. The number varies according to the manufacturing week rota. The Clinical Chemistry service is issued every two weeks as they are high volume tests. Lower volume tests may be bimonthly or even quarterly if they are highly specialised. |
| Q: |
How does the team at Birmingham Quality obtain expert advice when it is required e.g. when establishing an EQA programme for a new / niche analyte? |
| A: |
The EQA Programme Directors at Birmingham Quality obtain advice and guidance from the Steering Committee on Quality Assessment in Clinical Chemistry and the service's associated Specialist Advisory Group (SAG). Each UK NEQAS service has a dedicated SAG comprising subject matter experts, service users (clinical and technical) and a National Quality Assurance Advisory Panel (NQAAP) observer. |
| Q: |
Why isn't a low, medium and high concentration of analyte included in every distribution of EQA specimens? |
| A: |
Internal Quality Control (IQC) in laboratories covers the entire concentration range of the analyte being measured and provides laboratories with 'real time' information about the performance of their assay. EQA is not intended to duplicate information provided by IQC data but instead gives additional, retrospective data on performance. This includes an indication of a laboratory's assay bias, consistency of bias and accuracy for a particular analyte. |
| Q: |
Where do you obtain the clinical materials that are distributed through your EQA services? |
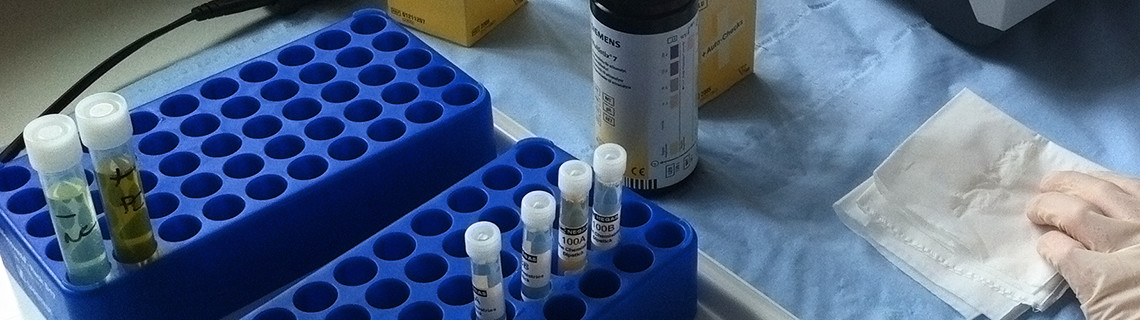
| A: |
Birmingham Quality uses genuine human material (plasma, serum, urine, faeces and fluids) wherever possible. We obtain the vast majority of our serum and plasma donations from NHS Blood and Transplant. We also distribute patient donations and purchase other material from a select number of commercial suppliers. Some matrices (faeces, sweat etc.) are prepared in house at our production facility in Birmingham where exogenous analyte is added at clinically relevant concentrations. |
| Q: |
How should I store the EQA specimens? |
| A: |
All packages dispatched from Birmingham Quality should be opened immediately upon receipt; an instruction to do so is clearly displayed on the outside of each envelope or parcel. Wherever possible, the specimens should also be analysed immediately upon receipt. Where this is not possible, and storage conditions have not been specified on the accompanying results document, serum, urine or plasma-based EQA material should be frozen at -20℃ or below as soon as is practicably possible after receipt. On the day of analysis, frozen specimens should be thawed completely and carefully at room temperature for no more than 2-3 hours. Refrigerated specimens should be removed from the fridge and brought to room temperature for approximately one hour before analysis. Each specimen should be mixed thoroughly by gently inverting the tube several times prior to analysis. For other specimens (whole blood, human faeces, artificial faeces and artificial sweat) where storage conditions have not been specified on the results document, these should be stored at +4℃ until the day of assay. |
| Q: |
How stable are the EQA specimens? |
| A: |
EQA specimens do have a finite shelf-life but they are all fit-for-purpose. Instructions explaining how the EQA specimens should be stored can be found on the accompanying results document but the best thing is to analyse straight away or store as you do for your clinical samples. The same EQA material is often distributed throughout an EQA programme on multiple occasions. The results obtained are compared to assess sample stability and variability within and between methods. Birmingham Quality also undertakes occasional formal degradation studies where required. Stability data of method means are often published in Scheme Commentaries or Birmingham Quality Annual Reviews and are also available on request for specific situations. |
| Q: |
How does Birmingham Quality assess the homogeneity and stability of the EQA specimens that they provide to participants? |
| A: |
All EQA material distributed by Birmingham Quality is prepared according to the highest standards and is subject to rigorous quality assurance processes. Participant data such as within-method variability of results (%CV), is used to assess specimen homogeneity. The same EQA material is often distributed throughout an EQA programme on multiple occasions. The results obtained at each Distribution are compared to assess sample stability and variability within and between methods. Birmingham Quality also undertakes formal degradation studies where required. Stability data is published in Birmingham Quality Annual Reviews and is also available on request. |
| Q: |
We are seeing “matrix effects” with Birmingham Quality EQA specimens. What should we do? |
| A: |
Occasionally, EQA material can exhibit “matrix effects” with some assays but please be reassured that we do take steps to minimise such effects. We also take any unavoidable matrix effects into account when “scoring” participants' EQA results. Our friendly Science Team will be happy to talk you through any problems you may be having. Before contacting us, we would advise you to examine your EQA reports to check if everyone using your method is seeing the same phenomenon and also whether you get quantitative recovery on specimens to which exogenous analyte has been added, even if your assay behaves differently with endogenous materials. A guide to interpreting your report can be viewed via the online Results and Reports Service by selecting the blue †Your username and password will be required to access this information. |
| Q: |
How do I return my EQA results to Birmingham Quality? |
| A: |
When you sign up to become a member of Birmingham Quality, you are given a unique Laboratory ID number and a password which will give you full access to Birmingham Quality’s online services. From the Birmingham Quality homepage:
Birmingham Quality has teamed up with the National Pathology Exchange (NPEx) to enable Participants to return their results directly to Birmingham Quality in the same way as they send results to their service users. If you are a user of the NPEx service and would like to use it to return your EQA results to Birmingham Quality, please contact us. |
| Q: |
My laboratory is unable to return results for the current Distribution of EQA specimens. What should I do? |
| A: |
Birmingham Quality actively encourages Participants to achieve a return rate of 100%. However, we are aware that it is not always possible to return results on time and sometimes not at all. We have established a mechanism by which Participants can inform us that they are unable to return results without this incurring a penalty for poor return rate. If you are unable to return a result for any reason, please enter XPL in the results field and provide an explanation in the comments box. If you are unable to return any results at all for a Distribution, before the Distribution closes for results entry, please log in and tick the box at the top left of the Results Entry screen (labelled: Tick this box if you have no results at all for this distribution; you must provide a brief explanation in the comments box below). Ticking this box will enter XPL in the results entry fields for all specimens and all analytes at that Distribution. Again, please provide a brief explanation in the comments box before clicking on the green Submit button. If results later become available, it is possible to submit them online for a limited period of time after the Distribution's closing date. See also: Can I return results after the closing date for the Distribution? and Whilst reviewing my EQA report, I have noticed I had submitted an erroneous result to Birmingham Quality. Can I amend it? 1 late and 1 amended result is allowed every 12 months. It is especially important for laboratories to return EQA results when their assays are performing below par. If you no longer wish to be part of the EQA service in question, no longer offer a service for particular analyte, or are having service issues, please contact Birmingham Quality. |
| Q: |
Can I return results after the closing date for the Distribution? |
| A: |
Birmingham Quality does accept late results (with the exception of POCT schemes) for a limited period after a Distribution has closed. Late results can be entered online in the usual way after the closing date and at any time up until the close of the next distribution. The data entry page will show (Amendment / Late request) after the Distribution number. Your results will be incorporated into your laboratory's cumulative data and a new report marked LATE will be uploaded to the web along with the current report. 100% participation with EQA services is expected, however 1 set of late and 1 set of amended results is allowed every 12 months. The number of LATEs are logged on the Participation Summary page. Birmingham Quality monitors the number of times a laboratory uses the XPL. 1 late and 1 amended result is allowed every 12 months. |
| Q: |
I wish to update the EQA results I have submitted to Birmingham Quality. The Distribution has not yet closed for results entry online. What do I do? |
| A: |
Results submitted using the online Results and Reports Service can be amended at any time before the closing date for the Distribution. Simply log in to the online Results and Reports Service with your username and password, make any changes and click on the green Submit button. |
| Q: |
Whilst reviewing my EQA report, I have noticed I had submitted an erroneous result to Birmingham Quality. Can I amend it? |
| A: |
For the vast majority of Birmingham Quality's EQA programmes, Participants may submit a request to amend erroneous results for a limited time period after the closing date for the Distribution. Log in to the Results and Reports Service in the usual way, using the drop down, select the Distribution number for which you wish to request an amendment and click on the 'Results' button. A screen showing the previously submitted results will be displayed. The Results Entry Screen will show (Amendment / Late request) after the Distribution number. Simply edit the affected results, provide an explanatory comment in the box at the bottom of the screen and click on the green Submit button. Birmingham Quality will only authorise amendments that have arisen due to non-analytical errors (such as result transcription errors) and only if they are accompanied by a valid explanation which the requester should provide in the comments box. The requester should also include their name and contact details in case any Birmingham Quality requires additional information in order to action the request. Requests to amend results for Distribution n may be submitted at any time after the closing date for Distribution n and at any time up until the closing date of Distribution n+1. It is essential that EQA reports are reviewed promptly after publication as Birmingham Quality will not consider requests to amend results from Distributions earlier than Distribution n. Requests to amend results are monitored and logged on the Participation Summary page of EQA reports. Any single Laboratory ID can amend results without penalty on one occasion in a 12 month period. |
| Q: |
I submitted a request to amend my laboratory's results but the request has been turned down. Why? |
| A: |
Birmingham Quality will only accept requests to amend EQA results where the erroneous results were due to non-analytical errors. Requests for amendment should always be accompanied by an explanation. The authorisation of amended results is at the discretion of the EQA Programme Director. Requests to amend erroneous EQA results caused by analytical errors and which could also have affected patient results (e.g. dilution errors) will not be authorised. Amendments will only be allowed for the previous month's report(s). The Amended report will be published in the usual on-line place when the Reports for the current distribution are published. |
| Q: |
I have entered in Late results on-line. When can I see my updated report? [The answer is also true for amended results] |
| A: |
Because we receive quite a few Late results, we cannot be processing and reprocessing data every time a new set of results comes in. Because you will have a Report on-line, albeit without your own data, which will contain all the method means and targets etc., you will be able to see where you lie. We download all Late results for Distribution n when we download the results for Distribution n+1. The revised Report for Distribution n will be published at the same time as Distribution n+1 and obviously Distribution n+1's Rolling Time Window scores will have taken into account the late results. This is the only practical approach to handling Late and Amended results. |
| Q: |
How should I report very low or very high EQA results to Birmingham Quality? |
| A: |
Please report your results exactly as you would report them to your service users. Our online Results and Reports Service allows the use of < and > (less than and greater than) symbols but please do not leave a space between the symbol and the numeral. For example, if the result obtained is “less than 5”, please enter your result as <5, not < 5. If there is no result due to technical problems, then enter result as XPL and add a comment in the comment box on data entry. Please do not enter results as 0 (zero) unless this is how you would usually report the result to your clinicians. There is no need to request repeat specimens if the result obtained is < or > (less than or greater than). If the result obtained is incompatible with life or the material generates an error code on your analyser, contact Birmingham Quality for advice. |
| Q: |
I have an Icteric / Haemolysis / Lipaemic index flag against one of my EQA specimens. Should I still report my results to you? |
| A: |
For the UK NEQAS for Serum Indices service ONLY, please report the actual value obtained for the analyte specified on the results document, AND using the boxes provided, record the cut-off concentration for each of the different serum indices (Haemolysis, Icterus, Lipaemia) used to determine whether or not the analyte would be reported. For all other Birmingham Quality EQA services, please report your results exactly as you would do for clinicians. e.g. if the index flag would 'knock out' the results, and prevent you from reporting them to the requesting clinician, please enter the result as XPL (explanation provided), and provide any comments that you would usually append to the results in the comments box on the online results entry screen. |
| Q: |
What does a Birmingham Quality EQA report look like? |
| A: |
A sample report can be viewed and downloaded from our website. Go to the Birmingham Quality homepage, click on the blue 'i' icon and select the EQA programme of choice from the list displayed. A link to an example report can be found below the list of analytes. Educational videos for individual elements of a report and a “Guide to Interpreting Your Report” can be viewed under the Education button via the online Results and Reports Service†. †Your username and password will be required to access this information. |
| Q: |
What is the turnaround time from a Distribution closing to receiving a report? |
| A: |
Birmingham Quality aims to have the report for any given Distribution published and available online as soon as possible after the closing date stated on the results document; usually within 48 - 72 hours. For our regular monthly Schemes, the report for Distribution n will be available no later than the dispatch date for Distribution n+1. Where a special study has been undertaken as part of a Distribution, the turnaround times may be a little longer. Our fortnightly general Clinical Chemistry Scheme we aim for the 48 - 72 hours after the closing date, but the next set of specimens will already have been dispatched. |
| Q: |
How do I view my EQA reports online? |
| A: |
When you sign up to become a member of Birmingham Quality, you are given a unique Laboratory ID number and a password which will give you full access to Birmingham Quality's online services. From the Birmingham Quality homepage, click on the orange graph icon labelled 'Login to Results and Reports'. You will be prompted for your Username (Laboratory ID number) and your password. Click on 'Sign in'. This will take you directly to your own area of the online Results and Reports service. Locate the EQA programme for which you wish to submit results in the list of EQA programmes displayed. Use the drop down menu to the right hand side of the EQA programme of choice to select the Distribution number for which you wish to view your report. Click on the 'Report' button. This will display your PDF Report for the Distribution of choice. |
| Q: |
How do I navigate around my EQA report? |
| A: |
Birmingham Quality EQA reports are published online in PDF format. We recommend that you view your report online as this allows you to take advantage of the hyperlinks embedded within the report to navigate seamlessly between the different sections. The bookmarks on left hand side of the report can be used to quickly access different sections of the report. It is also possible to use the page up / down buttons on your keyboard, or the scroll function on your mouse, to move to the next / previous pages on the report. Please do not use the BACK button to review previous pages of the report as this will return you to the Results and Reports page. |
| Q: |
Can I view historical EQA reports for my laboratory? |
| A: |
Log in to the the online Results and Reports Service using your username and password. Your laboratory’s two most recent reports can be accessed quickly using the blue buttons labelled with the Distribution numbers situated to the right of the relevant EQA programme. Earlier reports can be viewed by selecting the Distribution number of choice from the dropdown menu and then clicking on the grey Report button to the right of the relevant EQA programme. |
| Q: |
How do I interpret my EQA report? |
| A: |
Educational videos for individual elements of a report and a “Guide to Interpreting Your Report” can be viewed under the Education button via the online Results and Reports Service†. †Your username and password will be required to access this information. |
| Q: |
Does Birmingham Quality remove ‘outliers’ when analysing EQA data? |
| A: |
Healy trimming is used to identify and trim outliers; removing them from the calculations of statistical data such as means, standard deviations etc. The trimmed results will still be displayed on histograms etc. but will not have contributed to the calculation of statistical data. If one of your laboratory’s results is an outlier, the result will still be used in the calculation of the A, B and C scores for your laboratory which are then used to assess your performance. It is therefore essential that EQA reports are reviewed promptly after publication as Birmingham Quality will only accept requests to amend results for Distribution n up until the closing date of Distribution n+1. |
| Q: |
What is the target (assigned) value for a specimen? |
| A: |
Assigned values used are the best estimate of the true value and are usually consensus values. The assigned value can be the All Laboratory Trimmed Mean (ALTM), the method principle mean (GLTM), method laboratory trimmed mean (MLTM) or weighed-in level mean (XALTM). In some services the mean of one method or method principle is used as the target value, for example enzymatic methods for serum creatinine and mass spectrometry methods for the steroid hormone service. Participants need to be aware of the greater statistical “uncertainty” in those situations where the contributing number of data points is small. Note: The Target Value may not be the absolute “true” value to the n’th decimal place, either because “truth” can never be truly established, or it is the best value that we can obtain at a cost that Participants will be prepared to pay for. |
| Q: |
There is no target value displayed on my EQA report. Why is this? |
| A: |
If the target value is the method lab trimmed mean, we need to have a minimum number of labs using that method before we can provide a robust target. Also, we may not have a method allocated to your laboratory (you would be allocated a method code of UUU). When the analyte concentration is low and participants are reporting < results, the target may not be shown as there may not have been sufficient returns to obtain a robust target. This would also occur conversely if the analyte concentration was high and > results were reported. Note: the statistical Uncertainty around means calculated from small data sets can be rather large. |
| Q: |
What is a Penalty Box Plot and how do I interpret it? What do different domain patterns mean? |
| A: |
A penalty box plot is a graph of Bias ‘B’ score against Consistency of Bias ‘C’ score showing participants’s scores and boxes indicating all data, method data and acceptable limits of performance. Optimum performance would be to have a B score of 0 and a very low C score. If your method displays a tight cluster of B and C score co-ordinates it means it is a precise method and there will be a low between-laboratory CV. The position of the ‘box’ above and below the 0 bias line, depends on the target value used. Where the target is the MLTM the box should straddle the 0 line. Where the target is ALTM the box may be above or below the 0 line if there is an inherent method bias to the assay |
| Q: |
What is a Graphic Equaliser Plot? |
| A: |
A graphic equaliser plot is a horizontal chart of box & whisker plots containing data or scores for different analytes in a Scheme. When a single parameter is used to assess performance it is, by definition, a measure of Total Error (and would be an A score if one were calculated). It is of the same format as that used for methods within an analyte. The circle shows individual lab data. |
| Q: |
Why do you use a six month rolling time window when calculating performance data? |
| A: |
For many services, a six month time window gives 18 data points, which provides enough data to calculate meaningful, cumulative statistics that are relevant to the current laboratory performance. It is well judged compromise between having too much emphasis on current performance and historical performance. |
| Q: |
Why does the data on an EQA report display a greater number of decimal places than we would clinically report for an analyte? |
| A: |
For statistical reasons, we report specimen results data to participants to one more decimal place than that reported by the majority of Participants. You should continue to report your EQA results to Birmingham Quality to the same number of decimal places as you would to your service users (clinicians). |
| Q: |
We reported a value of 17 for analyte X. The target value was also 17 and yet our Specimen % bias is -2.2%. Why is this? |
| A: |
The target value displayed on your report, although displayed to the nearest whole number, actually has significant figures after the decimal point. In this case, the target value is actually 17.376... which is why your Specimen % bias is -2.2%. |
| Q: |
For the UK NEQAS for Acute and Chronic Kidney Disease, we obtained a Creatinine result similar to the target value but we scored poorly for the estimated GFR. Why is this? How should we investigate? |
| A: |
On the results document which accompanies the EQA specimens we supply gender, ethnicity and date of birth information for each of Patients A, B and C. Check that the correct gender, ethnic group and age for each Patient (A, B and C) have been entered correctly on your Laboratory Information Management System (LIMS). Booking in the specimen using the wrong gender, ethnic group or age will have a significant impact on your calculated GFR result and consequently, adversely affect the score you receive for eGFR on your EQA report. If upon checking, the gender, ethnic group and age information has been entered onto your LIMS correctly, please check that the equation being used to calculate the eGFR is correct. |
| Q: |
What is the 'expected eGFR' result displayed on my report for the UK NEQAS for GFR Estimations? |
| A: |
For each of Patients A, B and C, we provide an Expected eGFR calculated using the Creatinine result obtained by your laboratory, and the gender, ethnicity and date of birth information we supplied with the specimens. The Expected eGFR result provided by us should match the eGFR result returned by your laboratory. Any discrepancies should be investigated immediately. |
| Q: |
My organisation will shortly be investing in new analytical technology. Can Birmingham Quality provide us with information about the EQA performance for a specific analyser / manufacturer to assist us in our decision-making? |
| A: |
Birmingham Quality can provide a range of analyser / manufacturer-specific data in a variety of different formats. Facilities exist for reports to be based on a particular method rather than an individual laboratory. This has the same functionality in terms of data analysis and presentation, but individual results are replaced by the trimmed method mean. These reports are of value to participating manufacturers for monitoring their products, and to laboratories evaluating methods or undertaking a tendering exercise. Method reports may be requested on an ad hoc basis or received routinely by certain types of participant. Those interested should contact the relevant service Organiser to discuss their requirements in detail. |
| Q: |
How do I log out of the online Results and Reports Page? |
| A: |
Log out of the online Results and Reports service by closing your browser. |
| Q: |
What is the ALTM and how is it calculated? |
| A: |
The ALTM is the All Laboratory Trimmed Mean. This is a statistically trimmed mean of all the results provided by all participants for a single Specimen / Analyte combination. The ALTM includes data from all of the different methods and kits in use by the Participants. Healy trimming is employed to remove any obvious outliers prior to the calculation of the ALTM. Healy trimming incorporates Downton's estimates of standard deviation (SD) and is used to produce an unbiased estimation of the SD. |
| Q: |
Why do you use the ALTM or other consensus mean as the target value for EQA data, rather than using a reference method result as the target value? |
| A: |
Reference methods can be very expensive and it is difficult to ensure that reference method values can be made available in a timely enough manner to accompany the data from freshly-prepared EQA specimens. We, therefore, do not obtain reference method values on our EQA materials as matter of routine. We do however, where possible use reference method values as part of a wider protocol to validate the target value assigned to our EQA materials. |
| Q: |
What are the A, B and C scores and what do they mean? |
| A: |
The A, B and C scores are calculated over a rolling time window, combining data from a wide range of specimens dispatched during recent distributions (typically over the preceding six months). The A, B and C scores give you an indication of your laboratory's average performance over time. The A score is an Accuracy (total error) score and tells you, on average, how good your overall performance is. It takes several factors into consideration including bias, consistency of bias and the degree of difficulty associated with measuring the analyte at different concentrations. The A scores are transformed so that they are comparable across different analytes. The B score is a Bias score and tells you, on average, how far away your laboratory is, from the 'true' value for the concentration of analyte in the specimen. The B score is the (trimmed) mean of the individual specimen % biases in the rolling time window. The C score is the Consistency of bias score and provides an indication of how variable your laboratory's bias has been during the rolling time window. The C score is the (trimmed) standard deviation of the individual specimen % biases in the rolling time window. An explanation of the A, B and C scores can be found in the ABC of EQA training video under the Educational button on the Results and Reports website. You will require your username and password to access this information. |
| Q: |
How is the A score calculated? |
| A: |
An explanation of the A, B and C scores can be found in the ABC of EQA training video under the Educational button on the Results and Reports website. You will require your username and password to access this information. |
| Q: |
How is the B score calculated? |
| A: |
An explanation of the A, B and C scores can be found in the ABC of EQA training video under the Educational button on the Results and Reports website. You will require your username and password to access this information. |
| Q: |
How is the C score calculated? |
| A: |
An explanation of the A, B and C scores can be found in the ABC of EQA training video under the Educational button on the Results and Reports website. You will require your username and password to access this information. |
| Q: |
What is the Standard Uncertainty and how is it calculated? |
| A: |
The Standard Uncertainty displayed on your EQA report is the uncertainty associated with the target (assigned) value used for an analyte / specimen within an EQA programme. It is calculated using the formula: Standard uncertainty = 1.25 ' (SD/?n) |
| Q: |
What is the difference between a green diamond and a green circle on my report? |
| A: |
The Specimen-level statistics are diamonds (green), triangles (yellow and also showing direction of bias) and double triangles (red and also showing direction of bias). The rolling time-window statistics are circles, again RAG rated and are derived from data that spans a number of Distributions. The different shapes help you navigate your way round the reports. |
| Q: |
At what level of B score and C score do the icons change colour? |
| A: |
ABC score limits are set once there is enough robust data from participants to allow reliable levels to be determined. ABC score limits are set at different levels, giving rise to the 'traffic light' system of scoring; there is one limit for the green,yellow and red traffic lights, this allows the scoring to go from green to yellow to red depending on the performance. All ABC score limits are set by the service Organisers. The B and C score limits are analyte-specific, but the limits we use for A scores is constant. Green is less than 100 and Red is more than 200. |
| Q: |
Why is the All Laboratory Trimmed Mean (ALTM) or the Group Laboratory Trimmed Mean (GLTM) used as the target value when my results are always closer to my method mean? Am I being unfairly penalised? |
| A: |
The target value is our best estimate of the truth and are usually consensus values. Extensive studies have been performed to decide which target value is most appropriate for use within each service (ALTM, GLTM, MLTM, XALTM). We are happy to investigate any suggestions or disrepencies between your results and the target values, however if you are not achieving the same results as your peers it could be a sign that there is an issue with your assay or method specifically and not really the target value against which you are being compared. |
| Q: |
My EQA performance is consistently poor. What should I do? What will happen next? |
| A: |
This is a full topic in its own right. Simplistically you should already be aware from previous reports what the trends of performance are. This won't have come out of the blue. You should have an in internal SOP as to what actions you should take. You should record in your QMS the fact that you are outside limits or have been flagged as such on reports or have been sent a separate email / letter. There isn't a one-size-fits-all answer, but we would be happy to talk you through your particular situation. We are required to report to the relevant NQAAP - Chemical Pathology or Microbiology (and any successor structure) on laboratories whose performance scores move outside acceptable limits on a set number of occasions within the scoring time window, or who fail to return sufficient results. The performance of each laboratory identified is then reviewed in association with any correspondence between Organiser and the participant, and a decision is made on further action. This may be just to monitor, to stimulate dialogue between Organiser and participant and monitor improvement in performance, or to suggest that the 'Panel' Chairman should make contact. The latter course of action is relatively rarely undertaken and begins with a first Panel' letter' inviting the participant to make contact to discuss action to correct the poor performance. If a satisfactory response is made and improvement in performance ensues, no further action is taken. If poor performance persists or no response is made, then a second Panel letter (direct from Panel Chairman to Head of Department with lab code disclosed) is written requesting that decisive action is taken to re-establish satisfactory performance; this may include a site visit by Panel members. If this fails, the Joint Working Group may take further action. Where poor performance is purely method-related (eg all users have a large positive or negative bias), Organisers will normally work directly with manufacturers to assist with correction of any problem; analogous procedures are in place for apparently IVD-related problems (see JWG guidelines). |
| Q: |
I have received a Notification of Poor Performance but I am performing well compared to the other users of my method. Do I need to take any action based on these results? |
| A: |
Any red traffic light scores and a notification of poor performance must be logged in your quality management system and investigated. However, if your C score is low (i.e. your bias is consistent) and your method group has a similar B score, you may be in consensus with your method and you may need to raise this with your manufacturer. A response to a Notification of Poor Performance is required. Birmingham Quality can advise on any troubleshooting and investigation required if necessary. |
| Q: |
How do I add or delete an analyte or a whole scheme from my registration? |
| A: |
Either send us an email with your request or send a message via the comment box on the data entry screen. Not all changes can be made on line, especially when there are financial implications. |
| Q: |
How do I update the method information for a particular EQA programme or analyte? |
| A: |
Your method can be updated online.
If your method does not appear, please provide as much information as possible including details of the manufacturer and model of the analyser, reagent manufacturer and product code, and calibrator manufacturer and product code. Please provide this information in the comment box associated with the relevant analyte. |
| Q: |
How do I add a sub-method? |
| A: |
Sub-methods are not available for all methods. To select a sub-method the main method needs to be selected from the drop down menu before the sub-method can be chosen. |
| Q: |
We have no rolling time window scores (A, B and C scores) displayed on our EQA reports? Why is this? |
| A: |
Laboratories are only provided with rolling time window (aka cumulative) statistics after a certain number of numerical results have been returned. Often this is a minimum of 7 data points (which would usually be in the third Distribution). We reset the stats after a method change so that your stats reflect only your new method. |
| Q: |
We are looking to change our method / analyser and would like information on the performance of different method groups. Can Birmingham Quality help with this? |
| A: |
Birmingham Quality can provide anonymous data showing how the different method principles / manufacturers perform. Please contact Birmingham Quality if this is of interest. |
| Q: |
What are 'method' and 'submethod' classifications? |
| A: |
Methods are separated by method principle and manufacturer. Sub-methods are where there are differences within a manufacturers method principle such as different calibration factors in use or different generations of reagent in use. Sub-methods will only be maintained where the differences cause a clear split in results obtained by a method. Where there is a natural progression from one generation to another, sub-methods may not be created. |
| Q: |
Why am I grouped by manufacturer rather than instrument? |
| A: |
Manufacturers have many different instrument combinations. Where the technology is the same across the different platforms, we will register participants based on the manufacturer. |
| Q: |
I'd like to report my EQA results in different units to those displayed on the results document / EQA report. Is this possible? |
| A: |
Participants should report their EQA results in the units used to report to clinicians and should not apply a conversion factor just for EQA. Birmingham Quality have Scheme units for analytes. These are the units used most frequently by laboratories. Units can not be altered by participants and any request should be made to Birmingham Quality via the comment box or email. |
| Q: |
Can I request extra / repeat specimens for a distribution from Birmingham Quality? |
| A: |
We advise participants to keep leftover EQA material for a short period after analysis (until they receive their EQA report) to prevent them having to request repeat specimens for troubleshooting purposes. Storage instructions are provided on the results document which accompanies the specimens. Extra specimens of the previous Distribution can be requested from Birmingham Quality for reasons such as troubleshooting poor EQA performance. Sometimes we are able to provide additional material for verification purposes but there will be a charge associated with this. Please contact Birmingham Quality for more information on the Schemes for which this is currently available. |
| Q: |
Can I have a repeat / replacement specimen? |
| A: |
For current, live distributions, we are willing to provide an occasional set of repeat / replacement specimens at no extra charge. |
| Q: |
Can I have a year's worth of back specimens to verify our new analyser? |
| A: |
We can provide back specimens where we have sufficient stock levels. Not all analytes are equally stable. You don't need usually need a full year's worth and we have set up Verification Packs to assist you. We do charge for these, therefore please contact Birmingham Quality for more information. EQA specimens do not 'validate' a new method, though they can be used in verification alongside clinical specimens. |
| Q: |
Why am I unable to obtain repeat specimens for some EQA programmes (e.g. UK NEQAS for Glycated Haemoglobins for HbA1c analysis)? |
| A: |
Wherever possible, we distribute fresh, genuine clinical material through our EQA programmes. For example, specimens distributed through the UK NEQAS for Glycated Haemoglobins for HbA1c analysis, are aliquots of fresh whole blood donated by volunteers with and without Diabetes. The useful lifespan of fresh whole blood such as this cannot be guaranteed beyond a week or so. We have therefore taken the decision not to provide repeat specimens for EQA programmes where the lifespan of the material is short. This is also true for other programmes where we use genuine clinical material and there is only a limited supply. |
| Q: |
How do I become an Assessor in the UK NEQAS for Interpretative Comments in Clinical Chemistry? |
| A: |
If you have successfully completed FRCPath Part 1, been participating as an Individual member (not as part of a Group) for six months or more, and have a Participant Time-window Score above the 25th centile, you can apply to be an Assessor. Please contact us for more details. |
| Q: |
I am a participant in the UK NEQAS for Interpretative Comments for Clinical Chemistry and am due to go on maternity leave. Is there anything I need to do? |
| A: |
Let us know and we will keep your registartion open, even if you will not be returning comments on a regular basis. |
| Q: |
I am a participant in the UK NEQAS for Interpretative Comments for Clinical Chemistry and have now moved to a new hospital / workplace. Is there anything I need to do? |
| A: |
If you are participating as an Individual (not as part of a Group), you can keep your existing User ID (BAnnnn) and password, but if your email address has changed, please let us know so that we can keep in touch. If your previous employer paid your subscription, and your new employer is happy to do so, please provide us with their details (Organisation name, address, UK NEQAS ID number etc.) so that we can invoice them when your subscription becomes due for renewal. It is also possible for you to pay for your subscription yourself, by cheque. We will need your home address so that we can send the invoice direct to you. Please note, that if you do not inform us of your new contact details, it is possible that there may be an interruption in your participation in the UK NEQAS or Interpretative Comments. If you were the main contact for a group participation at your old hospital, you need to make sure that you have shared your password with the new contact and they should change the password to a new one. |
| Abbreviation | Expansion |
|---|---|
| ALTM | All Laboratory Trimmed Mean |
| CV | Coefficient of variation |
| eGFR | Estimated Glomerular Filtration rate |
| EQA | External Quality Assessment |
| GLTM | Grouped Laboratory Trimmed Mean i.e. method principle mean |
| IQC | Internal quality control |
| MLTM | Method laboratory trimmed mean |
| UK NEQAS | United Kingdom National External Quality Assessment Service |
| NHSBT | National Health Service Blood and Transplant service |
| POCT | Point of Care Testing |
| SD | Standard deviation |
| ISO | The International Organisation for Standardisation |
| XALTM | Analyte specific defined target value e.g. weighed in or reference value |



 button on the top left of the Results and Reports page
button on the top left of the Results and Reports page button situated to the right hand side of the EQA programme for which you would like to update your method information
button situated to the right hand side of the EQA programme for which you would like to update your method information


